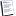 حل اسئلة للكيمياء ؟
حل اسئلة للكيمياء ؟
مين يعرف حل الاسئله ذي
5. The volume of a sample of nitrogen is 10.3 liters at 35°C and 0.970 atm.
What volume will it occupy at STP?
10.856 L
8.856 L
4.856 L
9.856 L
-------------
8. What is the mass of nitrogen gas in 1490.0 mL flask at STP?
0.864 g
1.864 g
5.864 g
4.864 g
هذا حليته طلع الجواب 0.932 !!
------------
9. What is the density of oxygen gas in 1L flask at 96 cmHg and 270 K?
3.826 g L-1
1.826 g L-1
4.826 g L-1
0.826 g L-1
------------
12. A mixture of three gases has a total pressure of 1920.0 mmHg at a certain temperature.
The mixture is analyzed and is found to contain 55 g CO2, 84 g CO, and 60 g Ar.
What is the partial pressure of CO ?
4.152 x10^3 mmHg
5.152 x10^3 mmHg
3.152 x10^3 mmHg
1.152 x10^3 mmHg
طلع لي الناتج 1001.74
-------------------
13. A mixture of 4 mol N2 gas and 6 mol O2 are in 5 L flask at 290 K, what is the total pressure?
45.56 atm
48.56 atm
50.56 atm
47.56 atm
----------------
14. Calculate the volume of PH3 at 20°C and 753.0 mmHg that can be prepared by additing a water to 1.48 g of Ca3P2.
Ca3P2(s)+6H2O(l)=3Ca(OH)2(s))+2PH3(g)
398.39 ml
391.39 ml
394.39 ml
395.39 ml
طلع لي الناتج 387.907

|